News Details

Parasites can induce drastic, alien morphological changes in their hosts. A textbook example of ant-parasite "shapeshifting" is a nematode that can turn the gaster (abdomen) of ants from black to shiny red, thus mimicking engorged "berries" that birds spot and gobble up. The parasite then completes its life cycle in the bird's gut.
KLI fellow Alice Laciny’s latest publication in the journal EvoDevo is the first comprehensive review of ant-changing parasite infections. Parasites affect adult ant morphology when they infect ants at the larval or pupal stage of the life cycle ("preimaginal infection"). The staggering diversity of parasites, hosts, and morphologies that occur as a result of preimaginal infection is the focus of her paper.
We sat down with Laciny to talk about the patterns that emerged from the collection, what they mean for the conceptual and empirical study of ant-parasite systems, and the future of "eco-evo-devo" (ecological evolutionary developmental) studies.

Cephalotes atratus infected by Myrmeconema neotropicum, with a berry-like gaster (left).
Lynn: Alice, you are known as an expert of “exploding ants,” the Colobopsis explodens worker ants that commit suicide as a last line of defense. How did you get from there to this work on parasite “shapeshifters”?
Alice: This review is actually a continuation of my PhD project on the caste morphology of Camponotini, aka the tribe that includes exploding ants. The different castes of this group sometimes look so different, one can easily mistake them for different species! While examining their colonies, I found that some of these ants were infected by nematodes. Then it hit me. Aside from normal caste –differences, some of these ants were so different because of the infections! However, when I looked around to find papers on morphological changes caused by parasite infections, I couldn’t find a general analysis, that is, a single collection that organizes or lists the relations between morphologies, parasites, and ants. There was a description here, a case study there, but nothing that synthesizes them all. So, I set out to write the paper I wanted to read.
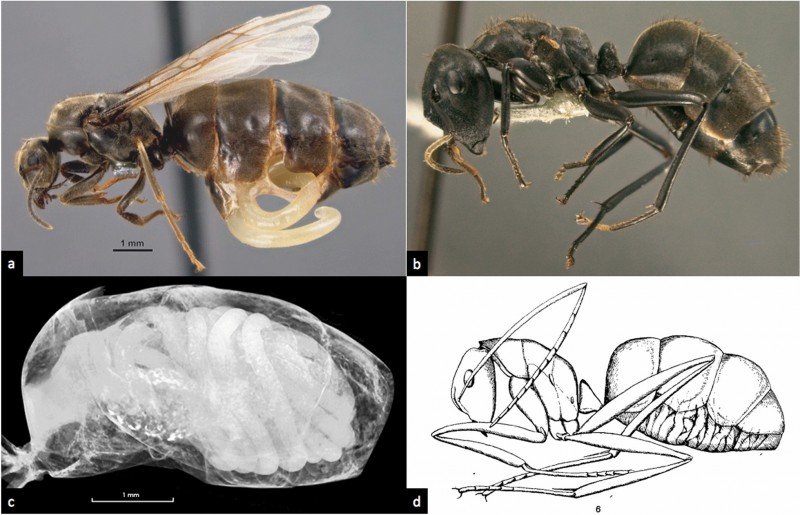
Panels b and c are the female castes of Colobopsis sp. nrSA, a Bornean “exploding ant” of the Colobopsis cylindrica group. They are hosts of the nematode Mermithidae. Panel a is a mermithid erupting from the gaster of another ant species-- Lasius niger.
Lynn: What are the sources of your review and how did you put order to the collection?
Alice: I went deep into museum archives, libraries, and online resources to dig out a diverse array of case studies in French, Spanish, Italian, German, and English. The oldest paper goes all the way back to 1747! The core of the project was to collect and compare these cases up to 2021. I’ve organized them in three ways: by the parasite organism, by the host ant species, and by common syndromes, that is, the morphological changes in ants induced by the parasite.
Lynn: That sounds like quite the task! Do you normally handle historical work like this?
Alice: Yes, it took a lot of time to trace these papers through citations and references. I speak German and English and learned French and Latin in school, so that helped quite a bit, but thankfully the scientific jargon is quite similar across these languages and Google translate was my friend! Archival work is actually quite normal in entomology. You can’t do anything without referencing historical records. Many species descriptions that we use today were recorded in the 1800s and we still go back to them repeatedly. In genetics, a 2004 paper is already too old, but it’s normal in my field to work on something as “recent” as 1804.

Labial gland disease in Formica polyctena (bottom two).
Lynn: What are some of the challenges you encountered while synthesizing these sources?
Alice: The most difficult part was figuring out a way to systematically handle inconsistencies. The authors have different opinions on what belongs in the same caste or species, and sometimes the same author draws inconsistent conclusions within their own work! That is why I added an extra section in the paper discussing how we can deal with this type of “unknown” or “uncertainty.” Who should we believe? How should we talk about or address these uncertainties? Where should I draw the line and say, ok, I can be certain up to this point but beyond that, I just don’t know? How we establish these judgments is a methodological and a philosophical problem. Interestingly, when I submitted an opinion piece about making well-informed interpretations and judgment calls, the reviewers had a problem with an abstract, general “how-to” guide. However, now that these discussions are contextualized with concrete examples in this big review, the reviewers had no complaints.

Mattesia sp. infecting Solenopsis invicta.
Lynn: I’m very curious about what emerged once you put the collection together. Did any unexpected patterns jump out? What did you make of them?
Alice: First of all, it was very surprising to see that many “modern” concepts such as niche construction and “eco-evo-devo” were already explicitly discussed in old papers. I was particularly delighted by a cluster of work developed in 1930s Germany that covered many of these theoretical issues.
About the specimens themselves, the diversity of organisms was fascinating, but the similarities in morphologies despite diverse causes were even more incredible. The pathogens I dealt with ranged from viruses to worms in a wide array of ant species. There was this huge diversity of insects and microorganisms involved and a diverse array of changes produced by the infections. Some of the changes are small quantitative shifts in size and shape while others are completely alien. I was astonished to find that in many cases, worms and viruses can produce similar phenotypes or “intercaste individuals” (individuals that cannot be assigned to castes anymore as they look too different from pre-existing categories) in different species!
Lynn: Tell me more! What does this all mean?
Laciny: First, the diversity of morphologies showcases the outer limits of what’s viable, that is, the extreme plasticity of organisms to change in such ways yet go about as living, breathing adults amongst their colony. Second, the similarities in morphologies show that there are general, underlying developmental mechanisms affected in similar ways by different organisms!
Lynn: So you are now able to study how things work by examining how they break and not break!
Alice: Exactly! These individual case studies, when put together, reveal a “natural experiment” of parasites interfering with different parts of ant ontogeny. This opens up all kinds of questions for contemporary (e.g., proteomic, genomic, etc.) studies of ant development, caste evolution, general ant evolution, etc. That is why while compiling these studies in an orderly manner, I noted not just what we knew about these systems, but more importantly what we still don’t know about the developmental pathways and mechanisms.
Practicing myrmecologists can use this as a roadmap to know what to look for and be aware of while they are examining their own specimens. Now that we know which changes are possible, this will get our alarm bells ringing. My hope is that we will be able to recognize the parasite-induced phenotypes and see that they actually pop up everywhere! In many cases, we accidentally describe a parasite-infected ant as a new species, and I have seen this type of problem riddled in the sources I examined.

Ant–parasitic Nematoda of the families Allantonematidae (a) and Seuratidae (b)
The paper can be summarized by this graphical abstract:

"the evolutionary and developmental factors mentioned in the literature surrounding parasite-induced phenotypes in ants; preimaginal parasitic infection (left box) may cause developmental perturbations (middle box), which are mediated by properties of individual ontogeny (clear arrows) as well as colony-level factors (grey arrows). This results in phenotypic changes to the host (right box), which may in turn mutually interact with further individual and colony-level evolutionary and developmental processes."
Lynn: To wrap up this interview, Alice, can you tell me why this is important for “eco-evo-devo” in particular, not just eco-devo, but eco-evo-devo?
Laciny: When parasites disrupt the normal development of ants, this is “eco-devo”—ecologically-induced development. Yet such interventions, as far as I know, are detrimental to individual fitness. When the impact is just on the workers, which are sterile, one might think that there are no direct evolutionary consequences as the condition cannot be passed on to future generations. However, the dip in fitness has consequences for the entire colony, thereby affecting the evolution of the entire population or species! We might see changes in fertility rates, in inter-colony competition, etc., and these are but one of the many ways evo can come into play. Since many parasites have multi-species hosts, with ants as intermediate or final hosts, the morphological changes might also affect ecological and thus evolutionary relationships.
Lynn: Alice, this work is incredible at all levels: conceptual, historical, methodological, and taxonomical. I really hope that it becomes a “go-to” paper for the many disciplines working on eco-evo-devo and entomology. Thank you so much for doing such important work and for accepting this interview! Read the article in full here: https://doi.org/10.1186/s13227-021-00173-2
Publication:
Laciny, A. Among the shapeshifters: parasite-induced morphologies in ants (Hymenoptera, Formicidae) and their relevance within the EcoEvoDevo framework. EvoDevo 12, 2 (2021).
https://doi.org/10.1186/s13227-021-00173-2
This interview was conducted and edited by Lynn Chiu.

